28565
Views & Citations27565
Likes & Shares
The present perspective is a synthesis of 97 published investigations in the setting of low cardiac output syndrome following pericardiostomy for large pericardial effusion and pericardiectomy for chronic constrictive pericarditis in the published literature. In this article, we reviewed the aetiopathogenesis, timings of operation, surgical approaches used in present day clinical practice, extent of pericardial decortication, requirement of cardiopulmonary bypass, useful investigative modalities to facilitate early recognition and therapeutic options of low cardiac output syndrome including use of intra-aortic balloon counterpulsation. Additionally, this review attempts to address many myths associated with operation and formulate guidelines to minimize the occurrence of low cardiac output syndrome thus improving survival.
Keywords: Chronic constrictive pericarditis, Hemodynamic monitoring, Intra-aortic balloon counterpulsation, Low cardiac output syndrome, Pericardial effusion, Pericardiectomy, Vasoactive therapies
INTRODUCTION
Despite improved diagnostic accuracy with echocardiography, computed tomography, aggressive preoperative stabilization, improvements in cardiac anesthesia, perioperative hemodynamic monitoring and advances in surgical techniques over the past 100 years, there remain several myths in the medical and surgical management of massive pericardial effusion and chronic constrictive pericarditis and there is no full-proof formula in the published literature to decide the optimal surgical approach for a given patient [1-10].
The low cardiac output syndrome (LCOS) was first described by Parr and colleagues in 1975. They used dye dilution to measure cardiac index (CI) and discovered that 25% of children after cardiac surgery have a cardiac index of less than 2.0 L/min/m2 [11]. Some 20 years later, Wernovsky et al. [12] demonstrated a similar incidence of LCOS in patients after the arterial switch operation.
Low cardiac output syndrome following pericardiostomy for cardiac tamponade was first described by Vandyke et al. [13]. These authors postulated that cardiac decompensation following tamponade decompression may be related to acute hemodynamic changes due to change in intravascular volume in the setting of dilated ventricles and changing systemic vascular resistance [13]. The Frank-Starling mechanism is improved initially by myocardial response to the release of constrictive pericarditis and improvement of right ventricular ejection fraction accompanying pericardiectomy but further increase of intraventricular volume causes increasing systolic wall stress, a reduction in stroke volume and low cardiac output.
However, Wolfe and Edelman [14] ruled out this hypothesis by this issue that complete recovery of LV function occurs in most patients after pericardiectomy. Two important predicting factors emerged from this study are abrupt increase in myocardial wall stress and time of chronicity of tamponade. Spodick [15] has revealed that this response may be related to the magnitude and the velocity at which the load develops.
Unresolved issues and controversies
Myth 1: Drainage of ascitic and pleural fluid following pericardiostomy and pericardiectomy lead to vascular collapse.
Myth 2: Routine usage of Digoxin in the preoperative period.
Myth 3: Median sternotomy and left anterolateral thoracotomy are equally effective approaches to achieve total pericardiectomy.
Myth 4: Cardiopulmonary bypass should be used as a routine to achieve complete pericardiectomy.
Myth 5: The recommended sequence of excision of the pericardium: from left ventricle àaorta à right ventricle à pulmonary artery à left atrium à right atrium.
Myth 6: For early recognition and timely intervention of LCOS which is the ideal investigative modality? Doppler echocardiography/tissue Doppler imaging/thermodilution catheter/pulmonary artery catheter/transesophageal echocardiographic monitoring/vigileoTM FloTracTM device/IKON.
Myth 7: Requirement and timing of institution of inotropic support: preoperative, intraoperative or post-operative for LCOS.
Myth 8: Role of intraaortic balloon counterpulsation in the management of post pericardiectomy/pericardiostomy LCOS.
METHODS
With these deficiencies in mind, we have analyzed the published literature to identify the described instances of LCOS and their management options following pericardiostomy for large pericardial effusion and pericardiectomy for chronic constrictive pericarditis. The search engines employed were Medline, PubMed, Google scholar, Cochrane database and Embase. The search included literature in all languages. This strategy yielded 97 investigations that provided best answer to these topics. We have then synthesized all these features to outline the rationale, pathophysiology of this paradoxical response, issue of concern and various surgical, non-surgical and myocardial supportive strategies which can be employed to achieve an improved clinical outcome.
Careful analysis of the published literature documents 4.1%-7.5% incidence of LCOS following pericardial decompression in patients with cardiac tamponade and between 24% to 28% in post-operative patients of pericardiectomy. The incidence of hospital mortality secondary to LCOS ranges between 24-28% in both groups of patients following either pericardiostomy or pericardiectomy which is unrelated to surgical procedure [15-34].
In order to understand the contributing factors that culminate in the LCOS, one must grasp the pathophysiologic aberrations that may impair cardiac function following pericardiostomy or pericardiectomy. The etiology of the LCOS is generally multifactorial [15-34].
It is well known to the clinicians that the hemodynamic hallmark of chronic constrictive pericarditis is impairment of ventricular diastolic compliance. On completion of a successful pericardiectomy, there are major fluid shifts from extravascular to intravascular compartments. We had previously demonstrated that this auto transfusion results in failure of Frank-Starling mechanism causing acute cardiac dilatation and this almost mimics acute LV dysfunction from volume overload. We often see some worsening of valve function due to acute stretching of the annuli resulting in functional regurgitation. Studies have shown, that massive ascites was a significant negative factor for survival [4,6,30-34]. Additionally, due to repeated mechanical compression during the process of pericardial mobilization, there is myocardial edema, which subsides over time. The universal phenomenon of hemodynamic improvement following pericardial decompression and paradoxical response with severe biventricular dysfunction despite cardiac filling and stroke volume in some patients have been observed by us and other investigators [4,6,30,31,33-35].
It has been established that constrictive pericarditis patients have more diastolic and systolic dysfunction than non-constrictive patients, including that caused by myocardial ischemia, stunning, atrophy or over dilatation. However, clinical presentation and outcomes of these patients with heart failure and depressed ventricular ejection fraction have not been studied in post pericardiectomy period. Therefore, the purpose of this study was to identify predictors of low cardiac output and mortality in decompensated severe heart failure in post pericardiectomy period [4,6,30-34].
The culprit pathophysiological mechanisms responsible for LCOS immediately following pericardiostomy/ pericardiectomy are not well understood. In order to understand the contributing factors that culminate in the LCOS, various theories have been proposed as the causative factor of LCOS, namely:
i. Occult systolic dysfunction which is masked by reduced chamber sizes and tachycardia. Pericardial decompression brings out the dysfunction. Vandyke and colleagues in 1983 postulated that that cardiac dysfunction following tamponade decompression may be related to acute hemodynamic changes related to change of intraventricular volume in the setting of dilated ventricles and changing systemic vascular resistance. The Frank-Starling mechanism is improved initially by myocardial response to the release of constrictive pericarditis and improvement of right ventricular ejection fraction accompanying pericardiectomy but further increasing of intraventricular volume causes increasing systolic wall stress, a reduction in stroke volume and low cardiac output [13].
ii. High levels of sympathetic tone and endogenous catecholamines during tamponade may mask pre-existing myocardial dysfunction which is accentuated by pericardial decompression. An imbalance between the sympathetic and parasympathetic output of the autonomic system with an apparent attenuation of sympathetic outflow following relief of cardiac tamponade, thereby unmasking occult left ventricular dysfunction, causing postoperative LCOS was hypothesized by Chamoun et al. in 2003 [36].
iii. Rapid over dilatation of the heart following pericardiostomy or pericardiectomy causing postoperative LCOS has been proposed by some investigators. This response may be related to the magnitude and velocity at which the load develops [8,15,37,38].
iv. Hamaya et al. [39] attributed this syndrome to myocardial stunning as results of changing intramyocardial blood distribution during tamponade surgery.
v. Wechsler et al. [40] proposed that subendocardial hemorrhage during tamponade drainage caused myocardial stunning and necrosis. The phenomenon of post pericardiectomy transient biventricular dysfunction in the absence of coronary artery disease was demonstrated by Ligero et al. [41]. Homogeneity of two-dimensional strain measurements shows that myocardial stunning may play a contributory role, because complete and uniform functional recovery was demonstrated in most patients [42].
vi. Immobilisation myocardial atrophy, myopericardial involvement by the same pathologic process, imperfect or incomplete decortication, remodelling of the ventricle, abnormal diastolic filling characteristics, worsening tricuspid regurgitation, and postoperative mitral regurgitation secondary to papillary muscle elongation have been variously implicated as the causative factors for LCOS [43-47]. Several investigators including ourselves have observed that regardless of the operative approach or extent of pericardial resection, a subset of these patients with chronic CCP will develop LCOS [4,6,9,10,30-34].
LOW CARDIAC OUTPUT SYNDROME
Definition and recognition
The low cardiac output syndrome in this context refers to the reduction in cardiac output that may occur following either pericardiostomy for massive pericardial effusion or pericardiectomy for chronic constrictive pericarditis. It is a well-recognized postoperative phenomenon that is quite commonly encountered following above-mentioned surgical intervention with dreaded consequences. Although no stringent diagnostic criteria exists, an accepted constellation of hemodynamic and physiologic alterations occur which alert the cardiac intensivist to its presence [15-34].
Low cardiac output syndrome following pericardiotomy or pericardiectomy was diagnosed if the patient required inotropic support (dopamine at 4-10 µg.Kg-1•min-1), dobutamine at 5-10 µg.Kg-1•min-1n, epinephrine at 0.01-0.1 µg.Kg-1•min-1, either isolated or in combination in the operating room or in the intensive care unit, to maintain stable hemodynamics in the absence of residual mechanical cardiac constriction, residual structural lesions such as significant valvular lesions and mechanical external compression after correction of all electrolytes or blood gas abnormalities and after adjustment of the preload to its optimal value. Low-output syndrome was also diagnosed if there was an increasing requirement of the above-mentioned inotropes with or without intra-aortic balloon counter pulsation along with afterload reduction with sodium nitroprusside. Patients who received less than 4 µg/kg/min dopamine to increase renal perfusion were not considered to have low output syndrome [15-34].
Accordingly, under the definition of low output syndrome after pericardiostomy and pericardiectomy, an integration of relevant clinical, laboratory and bedside echocardiographic criteria were used. The criteria for diagnosis were as follows: cold extremities, absent pedal pulses, decreased toe temperature, reduced systolic pressure, impaired renal function and oliguria (-1.h-1), metabolic acidosis, increased serum lactate levels >2.0 mmol/L, >2 h), low mixed venous oxygen saturation (<50%) and blunt sensorium [15-34].
Monitoring and diagnostics
The key to mitigating the LCOS in the post-operative period is early recognition and timely intervention. Many physiologic, hemodynamic and serologic variables can be assessed and re-assessed in order to follow a trend more valuable than any single point measurement or evaluation. In clinical practice, serial non-invasive, semi-invasive, and invasive monitoring strategies are available. It is important to appreciate that estimations of cardiac function, cardiac output and tissue oxygenation, based on the interpretation of standard clinical parameters and hemodynamic parameters such as the central venous pressure, heart rate and blood pressure are often discordant from measured values [15-34]. The use of adjunctive monitoring modalities is invaluable in making a timely and accurate assessment of cardiovascular function and the adequacy of tissue oxygenation [48,49].
Assessment of cardiac performance is of paramount importance in the management of patients undergoing pericardiectomy for constrictive pericarditis [15-34,53-56]. Doppler myocardial imaging is an echocardiographic technique that has the potential to enhance diagnostic performance available from Doppler blood-flow indices. However, we demonstrated in our previous investigation that TDI-derived mitral and tricuspid annular velocities are non-predictors of operative outcome in patients undergoing pericardiectomy [46].
Despite the accuracy of thermodilution technique for measuring cardiac output, it is invasive and there is an unclear risk-benefit ratio [57,58]. Recently, less invasive techniques such as transthoracic bioimpedence, pulse dye densitometry, LiDCO system, PiCCO-system (Paulson SG) have been developed for hemodynamic assessment [57-60]. However, the validity, practicability and accuracy of these techniques are not uniform [60].
Assessment of patient’s cardiac output and other hemodynamic parameters usually involves placement of a pulmonary artery catheter and performing thermodilution assessment [57,58]. This is an invasive procedure requiring balloon flotation of a catheter through the right heart and an elaborate protocol of intermittent pulmonary artery injection for thermodilution calculation. Secondly, surgical manipulation of the heart during pericardiectomy can make thermodilution, pulmonary artery, central venous monitoring and transesophageal echocardiography unreliable as monitors [30,33,34,57,58,61].
The emergence of new modalities of non-invasive haemodynamic monitoring have opened up newer frontiers for evaluation of such patients without the risk of invasive cardiac catheterization [59,60,62]. The FlotracTM sensor and VigileoTM monitor system introduced by Edwards life-sciences allows continuous measurement of cardiac output without requiring thermodilution or dye dilution. It bases its calculations on arterial waveform characteristics in conjunction with patients demographic data and does not require external calibration [30,57-63].
Therapeutic options for the low cardiac output syndrome
It is indeed impossible to pinpoint a specific causative factor for LCOS following pericardiectomy. Although high right atrial pressure and atrial fibrillation are associated with poor outcomes, we do not advocate an aggressive surgical approach to treat tricuspid regurgitation or atrial fibrillation at the time of pericardiectomy. It has been the authors practice to digitalize these patients in the preoperative period to control heart rate and minimize/avoid the chances of perioperative supraventricular arrhythmias, thus reducing the incidence of LCOS.
In our previous investigation on the effects of pericardiectomy via median sternotomy on intra- and post-operative hemodynamics by a semi-invasive device (VigileoTM monitor with FloTracTM sensor, Edwards Lifesciences, USA), we had demonstrated that despite decrease in right atrial pressure, systemic vascular resistance (SVR) and improvement in cardiac output, the stroke volume (SV) did not increase proportionately on completion of surgery. On the contrary, these parameters (i.e., stroke volume, stroke volume index) decreased from the preoperative levels immediately following surgery. Subsequently, the indexed SV continued to improve in the postoperative period and returned above the preoperative values at discharge. This transient depression of the stroke volume parameters in these patients could be multifactorial. It is well known to the clinicians that the hemodynamic hallmark of CCP is impairment of ventricular diastolic compliance [15-34]. On completion of a successful pericardiectomy, there are major fluid shifts from extravascular to intravascular compartments. Additionally, due to repeated compression during the process of pericardial mobilization, there is myocardial edema, which subsides over time [15-34].
A myriad of therapeutic strategies can be applied to support cardiac function and low cardiac output and include inotropic and afterload reducing agents, mechanical ventilation and mechanical circulatory support. The optimal timing for therapeutic interventions for the LCOS is prior to the onslaught of end-organ ischemic injury and the development of organ failure. Serologic markers of anaerobic metabolism such as serum lactate levels while generally indicative of inadequate tissue oxygenation are relatively late signs of cellular hypoxia, further emphasizing the importance of monitoring modalities such as venous and NIRS oximetry, vigileo monitoring which are discussed under monitoring. As mentioned above, the LCOS is an umbrella term encompassing any set of conditions leading to an imbalance of oxygen supply and demand. A deliberate evaluation of the cause(s) of compromised cardiac output must take place and consideration must be given to the impact of those lesions on ventricular loading conditions and function, as well as on heart rate and the conduction system. Strategies to optimize cardiac output and minimize oxygen demand will be reviewed [15-34,53-64].
Optimizing preload
A determination of where the ventricles reside on their pressure stroke volume curve is essential for determining the optimal ventricular filling pressure (central venous or right atrial and left atrial pressures). It is important to note that a given atrial pressure does not correlate with ventricular volume or stroke volume due to alterations in ventricular compliance. Furthermore, in the setting of LCOS following pericardiostomy and pericardiectomy, there is no correlation between right atrial and left atrial pressures, making it even more challenging to determine the optimal filling pressure for the left ventricle. Administering volume and objectively assessing the response provides some indication of where the ventricles reside on their pressure stroke volume curve. A prompt decrease in heart rate or increase in venous oxygen saturations or invasive blood pressure immediately following volume administration indicates that preload reserve is present, and that the ventricles are operating on the ascending portion of their pressure stroke volume curve. The lack of a response suggests that the ventricles are residing on the flat portion of their function curves. In this case, preload reserve is exhausted and inotropic and or afterload reducing agents are indicated to improve stroke volume and cardiac output [53]. Such an assessment of preload reserve although is of particular importance in patients undergoing cardiac surgery may not be applicable in patients undergoing pericardiostomy and pericardiectomy because of the phenomenon of auto-transfusion and cardiac dilatation in the setting of impaired ventricular compliance. Trial administration of intravenous fluids or blood in these patients may actually prove deleterious and monitoring of CVP alone (which is a static preload indicator) may not suffice for hemodynamic assessment [51,65,66].
Fluid responsiveness in perioperative and postoperative period in patients undergoing pericardiectomy may not be possible with CVP monitoring alone. In our previous investigations, we have demonstrated that there are massive fluid shifts with autotransfusion in this subset of patients undergoing pericardiectomy. In fact, massive ascites was significant negative factor for survival according to multivariate analysis [4,6,30,31].
Stroke volume variation (SVV) is the beat-to-beat change in stroke volume around the mean in one respiratory cycle. Previous investigators have demonstrated that a large SVV (>10%) in a mechanically ventilated patient indicate that the patient is likely to respond to fluid administration [30,31,53-66]. It has been the authors practice to insert a peritoneal dialysis catheter within the peritoneal cavity intraoperatively during pericardiectomy and drain the ascitic fluid over next 2-3 h to prevent sudden autotransfusion, increased preload and failure of the Frank-Sterling mechanism after surgery.
Manipulating systolic function
In our previous investigation, we had demonstrated a marked elevation of SVV (SVV>10%) in all patients at presentation in the operating room and during decortication. This possibly is due to decreased compliance of both ventricles secondary to generalized pericardial compression, dissociation between intrathoracic and intracardiac pressures and an interventricular “coupling” phenomenon, resulting in a septal shift [4,51,52]. Subsequently, despite restriction of fluid administration, there was only mild reduction of SVV throughout the postoperative period. Ideally, once constriction is relieved, SVV should have immediately dropped and become more dependent on blood volume. The above findings of SVV could be explained by the above explanations as well as our findings of statistically significant alterations in vasomotor tone as reflected by low SVRI following pericardiectomy. The third possibility of residual postoperative constriction in patients undergoing pericardiectomy via median sternotomy posterior to the phrenic nerves cannot be ruled out since the data shows borderline high SVV values in some patients even after surgery [51-53,64-66].
Once adequate preload has been established, inotropic support may be indicated. Many options exist with various side effect profiles and dose-dependent hemodynamic effects. Although not a classic inotrope, the calcium ion is essential to myofibril contraction. The myocardium in these patients is especially sensitive to changes in serum calcium levels. Intravenous calcium administration causes both increased contractility as well as increased smooth muscle tone in the peripheral vasculature. Thus, ensuring adequate levels of ionized calcium is essential in the management of patients following pericardiostomy and pericardiectomy.
Catecholamines are the mainstay of inotropic support. In brief, dopamine and dobutamine provide modest inotropic support with greater inotropy provided by epinephrine and norepinephrine. Dobutamine and epinephrine in low doses (
Manipulating afterload
Another strategy to improve cardiac output is to reduce ventricular afterload. The benefits of afterload reduction increase as systolic function wanes. Phosphodiesterase (PDE) type III inhibitors are an attractive agent for this purpose as they provide modest inotropic support with concomitant reduction of pulmonary and systemic vascular resistance. Additionally, they are less chronotropic, less arrhythmogenic and have less of an impact on myocardial oxygen demand than catecholamines. PDE type III inhibitors act by preventing the breakdown of cyclic adenosine monophosphate (cAMP). The accumulation of cAMP in vascular smooth muscle cells leads to vasodilation while in the cardiomyocyte it leads to improved contractility. In addition, PDE type III inhibitors do not rely on adrenergic receptors and are therefore immune to adrenergic receptor downregulation, which begins to occur within hours of exposure to endogenous and exogenous catecholamines [53].
The use of milrinone to prevent or treat LCOS although well documented in pediatric cardiac surgical and other cardiac surgical setting, it has not been studied in patients following pericardiostomy or pericardiectomy [53,67-71]. In a double-blind, placebo controlled trial of infants following pediatric cardiac surgery Hoffman and colleagues showed a 64% relative risk reduction of LCOS in the first 36 h following cardiac surgery in infants randomized to high dose milrinone (0.75 mcg/kg/min) compared to placebo, low or moderate doses [70].
Other agents useful for reducing ventricular afterload include the nitric oxide donor’s nitroprusside and nitroglycerin and calcium channel blockers. Nitric oxide works via cGMP causing vascular smooth muscle cell relaxation. Nitroprusside is a potent, readily titratable agent that vasodilates venous capacitance and arterial resistance vessels in a dose-dependent manner. Nitroglycerin is low doses vasodilates venous capacitance vessels while in higher does it also vasodilates arterial resistance vessels [53].
Levosimendan is a new cardioprotective inotropic agent having adenosine triphosphate dependent potassium channel opening and calcium sensitization of contractile proteins. It has mild phosphodiesterase inhibitory action and improves cardiac performance without activating the sympathetic nervous system [71]. It has been approved for management of acutely decompensated cardiac failure and for perioperative use in cardiac surgical patients with myocardial dysfunction [72,73]. There is no documentation in the published literature of its usage in LCOS following pericardiectomy.
Vasopressor therapy
Vasomotor paresis is characterized by a pathologic decrease in vascular tone, which increases venous capacitance and decreases SVR. Several agents may be used to restore adequate vascular function. Vasopressin, an endogenous hormone produced in the hypothalamus. Vasopressin acts on V1 receptors in the peripheral vasculature to cause intense vasoconstriction via activation of protein kinase C, ultimately leading to an influx of intracellular calcium. The rationale for using vasopressin as a therapy to treat refractory hypotension originated from the research produced by Landry and colleagues, which demonstrated that vasopressin levels were lower in adults with refractory vasodilatory septic shock [78]. It is important to note that augmenting SVR, while improving the mean arterial pressure, may cause a reduction in stroke volume and cardiac output, particularly in patients with impaired systolic function [74-78].
The usefulness of vasopressin in neonates with catecholamine-resistant shock following CPB has been documented by several investigators [74-78]. We have successfully used vasopressin in post pericardiostomy and pericardiectomy cases where conventional measures of catecholamine usage have failed [4,6,33,34,51]. The use of glucocorticoids for catecholamine-resistant shock for a period of 24-72 h is another strategy that may be of benefit following pericardiostomy and pericardiectomy [4,6,33,34,79-81]. Glucocorticoids may work through a number of mechanisms, including an increase in the expression of adrenoreceptors [53]. The use of glucocorticoids however is not without its challenges. In patients having post-pericardiectomy LCOS, the response to hydrocortisone does not appear to be related to the baseline cortisol level. In addition, the administration of high dose glucocorticoids may be associated with increased morbidity [79-82]. Post-operative hydrocortisone administration has been identified as an independent risk factor for the development of a catheter-associated bloodstream infection following cardiac surgery [79-82]. Although used clinically, the use of perioperative steroids in multicenter trials have not demonstrated any mortality benefit in multi-center studies [82]. Most recently, cumulative steroid exposure (7 vs. 4 days, p<0.001) has been shown to be independently associated with occurrence of infection in postoperative cardiac patients [79-86].
The role of positive pressure ventilation in the low cardiac output syndrome
Positive pressure ventilation is an invaluable tool in the armamentarium to treat the LCOS. Positive pressure ventilation (PPV) increases intrathoracic pressure thereby decreasing systemic ventricular afterload, which is of particular benefit to patients with impaired systemic ventricular systolic dysfunction. Another benefit of PPV results from the mechanical unloading of the respiratory muscles when cardiac output is limited. By mechanically unloading the respiratory pump, respiratory muscle perfusion requirements decrease and a limited cardiac output may be redistributed to other vital organs, including the brain and myocardium [86].
Mechanical circulatory support
Although the use of intra-aortic balloon counterpulsation (IABC) is universal in adults with acute left ventricular dysfunction after myocardial infarction or cardiac surgery, its use in patients undergoing pericardiectomy for chronic CCP remains sporadic [87-93]. If the cardiac output cannot be sustained by the currently available medical treatment, the next strategy may be to assist the failing heart by mechanical circulatory assistance. Intra-aortic balloon counterpulsation facilitates recovery of left ventricular function by decreasing left ventricular end-diastolic and left atrial pressure, thus helping the systemic ventricle and indirectly helping the pulmonary ventricle by the phenomenon of ventricular interdependence [93].
Although the use of balloon counterpulsation is universal in adults with acute left ventricular dysfunction after myocardial infarction or cardiac surgery, its use in patients undergoing pericardiectomy for chronic CCP remains sporadic [87-93]. The advantages of balloon counterpulsation over left atrial-aortic assist devices are the ease of application [87-93]. Other assist device like axial flow pumps and veno-arterial extracorporeal membrane oxygenation have been used as a salvage procedure [61,94]. Use of balloon pumping for supporting the failing myocardium also remains limited in children with chronic constrictive pericarditis [94]. This is due to technical difficulty in inserting balloons in infants or small children, along with the availability of such balloons, and inability to track rapid heart rates and narrow pulse pressures of children in shock. Additionally, complications like ischemia of the limbs, renal failure and mesenteric ischemia are greater for smaller children because of inappropriate lengths of the balloons [94].
Although today, the pediatric balloon catheters and pumping consoles have greatly evolved, there are commercially available devices suitable even for the smallest child and the early concerns of achieving effective counterpulsation in the highly elastic and distensible aorta of young children have proved unfounded [94]. Perhaps, the major concern that has hindered the widespread use of intra-aortic balloon in children in contradiction to adults is that, children are less likely to have preserved right ventricle and pulmonary function and may not be supportable with intra-aortic balloon. Extracorporeal membrane oxygenation and left ventricular assist device are the most prevalent means of mechanical circulatory assistance in such a clinical situation [92-94].
The timing and indications of balloon deployment is a matter of judgment. In patients who suddenly deteriorate after total pericardiectomy and are unresponsive to medical therapy, the decision to initiate IABC is relatively straightforward. The other clinical scenario would be in cases of progressive deterioration of ventricular function and unresponsive to adequate isotropic support [93]. The right ventricle is especially sensitive to changes in afterload, as it has significantly less contractile reserve than the systemic or left ventricle.
The insertable lengths of the commercially available intra-aortic balloon catheters are 16.5, 22.1 and 25.8 cm for 25, 34 and 40 cm3 balloons, respectively. The pediatric patients with preserved right ventricular and pulmonary function requiring mechanical circulatory assistance fulfilling the above mentioned mandated insertable balloon lengths may be candidates for intra-aortic balloon support albeit with 57% (n=4) dying despite using IABC.
Our search of the literature revealed seven patients with a failing circulation post-pericardiectomy treated with intra-aortic balloon support [87-93]. Tokuda et al. [20], Zhu et al. [21] have documented 11 more patients requiring intra-aortic balloon support post-pericardiectomy. In 2018, we reported 2 patients aged 18 and 19 years undergoing total pericardiectomy for chronic calcific pericarditis with systemic ventricular failure in whom the failing circulation was successfully re-established using IABC [93].
Role of cardiopulmonary bypass in the management of pericardiectomy
While cardiopulmonary bypass (CPB) may not be necessary for effusive or inflammatory pericarditis, it does all depend on how the patient tolerates cardiac manipulation; likely the most important reason to use CPB in order to facilitate a complete pericardiectomy because we know that this is more favorable in terms of long-term functional outcomes compared to a partial pericardiectomy. Studies in which CPB was associated with lower survival and higher risk including the Stanford series, demonstrate that this is a reflection of a more advanced disease process when CPB is needed [95-97]. So, we should not be reluctant to utilize CPB if needed to facilitate a complete resection with the thought process that this will lower survival. If it enables a more complete resection, this will undoubtedly impact patient's outcome more favorably compared to the use of CPB lowering survival. Additionally, in these patients, the use of CPB allows one to control these fluid shifts and ultra-filtrate some of this fluid off. So, this may be a concept that is not appreciated the use of CPB to avoid cardiac distension [4,6,30,31]. Although routine use of CPB to achieve total pericardiectomy was an issue of debate, it requires to be employed in special circumstances, namely (i) inadvertent damage to a cardiac chamber; (ii) cardiac operation, or previous partial pericardiectomy; (iii) presence of calcific pericardial “cocoon” encompassing all cardiac chambers; (iv) pericardiectomy following mediastinal irradiation; and (v) coexistent cardiac lesion [4,6,30-34].
CRITERIONS FOR DECISION-MAKING ON THE TIMING OF OPERATION, SELECTION OF SURGICAL APPROACH, ADEQUACY OF PERICARDIECTOMY AND THEIR RELATIONSHIP TO LOW CARDIAC OUTPUT SYNDROME
The clinical course of constrictive pericarditis is usually progressive and it is extremely difficult for the cardiologist to delineate the degree of pericardial constriction and myocardial restriction. The result of pericardiectomy are poor with dominant myocardial involvement and better with dominant constrictive element [6,30-34]. Early pericardiectomy is beneficial for patients with a central venous pressure between 12 and 15 mm Hg, RA pressure >24 mm Hg, hepatic dysfunction, renal dysfunction and massive ascites. Survival of patients with CCP following pericardiectomy is higher than without surgery [4,6,30-34,51].
Despite the effectiveness of surgery, there are disparate opinions regarding the role of corticosteroids in treating tuberculous pericarditis, timing of operation, surgical approach, extent of decortication and requirement of cardiopulmonary bypass [4,6,30-34]. The efficacy of pericardiocentesis in preventing CCP in pericardial effusion (serous/or hemorrhagic) has been inadequately investigated [14-17]. The terms “total”, “complete”, “extensive”, “radical”, “partial”, “subtotal” and “near-total” pericardiectomy have been variably used to describe the procedure, often without precise definition of the limits of pericardial resection [4,6,15-34].
Published reports attest to the unpredictable and variable pattern of CCP and lend support to radical decortication. In 2005, to define the limit of pericardial resection, total pericardiectomy was defined as wide excision of the pericardium with the phrenic nerves defining the posterior extent, the great vessels including the intrapericardial portion of superior vena cava and superior vena cava - right atrial junction defining the superior extent and the diaphragmatic surface, including the inferior vena cava - right atrial junction defining the inferior extent of the pericardial resection [4,33]. Constricting layers of the epicardium were removed whenever possible and the atria and venae cavae were decorticated in all cases in this study group. Pericardiectomy was considered partial if both ventricles could not be decorticated completely because of dense myopericardial adhesions or calcification [4,33]. Radical pericardiectomy was defined as removal of the entire pericardium over the anterolateral, diaphragmatic surfaces of left ventricle, portion of pericardium posterior to the phrenic nerve and the left ventricle and the anterior and diaphragmatic surfaces of RV until the atrioventricular groove leaving behind intact left and right phrenic pedicles [4,33].
Secondly, the importance of unrecognized constricting epicardial peel was described by Harrington in 1944 and successful pericardiectomy requires removal of all constricting layers including decortication of the ventricular epicardium [2]. In a study, Kolster et al. [54] demonstrated normalization of pressure volume loop as an indicator of operative success of pericardiectomy.
In 2005, we compared two surgical approaches used for the treatment of CCP, i.e., median sternotomy and conventional left anterolateral thoracotomy in 395 patients. The surgical approach was primarily based on surgeon’s preference and remained uniform [4]. However, the median sternotomy approach was preferred in the following conditions: (i) annular CCP; (ii) presence of a gradient between the superior or inferior venae cavae and right atrium of 2 mm Hg or greater; (iii) calcific pericardial patch compressing the RA and right ventricular outflow tract; (iv) extracardiac intrapericardial mass; (v) previous open heart surgery; (vi) circumferential ‘cocoon’ calcification of the pericardium; and (vii) recurrent CCP after partial pericardiectomy [4]. We demonstrated that the maximum benefit occurs after total pericardiectomy, which is best achieved through a median sternotomy and is very difficult through a conventional left anterolateral thoracotomy [4,6].
However, a left anterolateral thoracotomy was the preferred approach in cases of purulent pericarditis and effusive constrictive pericarditis because of the presence of concomitant pyothorax and the concerns of sternal infection [4,6,31].
In our previous study, we compared the outcomes after total versus partial pericardiectomy. Our study demonstrated that total pericardiectomy was associated with lower operative mortality and LCOS, abbreviated hospitalization and better long-term survival than partial pericardiectomy. Ascites, renal dysfunction, hyperbilirubinemia, high preoperative RA pressure (>24 mm Hg), atrial fibrillation, low ejection fraction (0.40 or less), pericardial calcification, tricuspid regurgitation, mitral regurgitation, partial pericardiectomy, thoracotomy approach and postoperative LCOS negatively affected survival [4]. In this study, the risk of death was 4.5 times higher (95% CI: 2.05-9.75) in patients undergoing partial pericardiectomy compared to total pericardiectomy [4].
Despite total pericardiectomy, the operative mortality rate was 7.6% in our series and 6% to 19% in several large series published after 1985 [4,6,16-34]. Unlike others, there was no correlation with age, tuberculous etiology and advanced NYHA symptoms on late survival, presumably because of young patient population and timely institution of chemotherapy and surgery [4,6,16-34].
Although the median sternotomy approach allowed a more radical clearance of pericardium overlying the right atrium and venae cavae including the cavo-atrial junctions, these areas usually are of little hemodynamic significance in the majority of patients. Furthermore, it is impossible to excise the portion of the pericardium posterior to the phrenic nerve using this approach [4,6,15-34].
Radical pericardiectomy via left anterolateral thoracotomy without cardiopulmonary bypass (UKC’s modification)
As enunciated above, the median sternotomy approach was the preferred option of the author (UKC) in the selected heterogenous group of patients undergoing pericardiectomy [4]. In an effort to decrease the hospital mortality rates and postoperative LCOS, the author proceeded to perform several technical modifications of the conventional left anterolateral thoracotomy approach to achieve further radical excision of the pericardium posterior to the phrenic nerve and diaphragmatic pericardium without utilizing cardiopulmonary bypass [4,6]. Thus, there were seven forces driving our decision-making towards improvement of the results after pericardiectomy via modified anterolateral thoracotomy.
· The desire to obtain improved operative exposure of the RV and RA by developing a new dissection plane between the posterior surface of the sternum and anterior surface of the pericardium.
· The desire to dissect the pericardium posterior to the phrenic nerve overlying the left atrium and postero-lateral surface of the left ventricle.
· The desire to develop a new cleavage plane between the diaphragmatic pericardium and diaphragm.
· The desire to minimise cardiac manipulation at the time of dissection by dividing the anterior and posterior pericardial flap in two halves, respectively.
· The desire to minimise postoperative autotransfusion by inserting a peritoneal dialysis catheter before surgical incision and placing it on gravity drainage intraoperatively.
· The desire to maintain oxygenation and hemodynamic stability during pericardiectomy via left anterolateral thoracotomy by placing an intercostal chest drain on the opposite side in case of right-sided significant pleural effusion.
· The desire to keep both groins prepared at the time of pericardiectomy via modified left anterolateral thoracotomy in case of inadvertent injury to the cardiac chambers and/or great vessels and urgent institution of cardiopulmonary bypass.
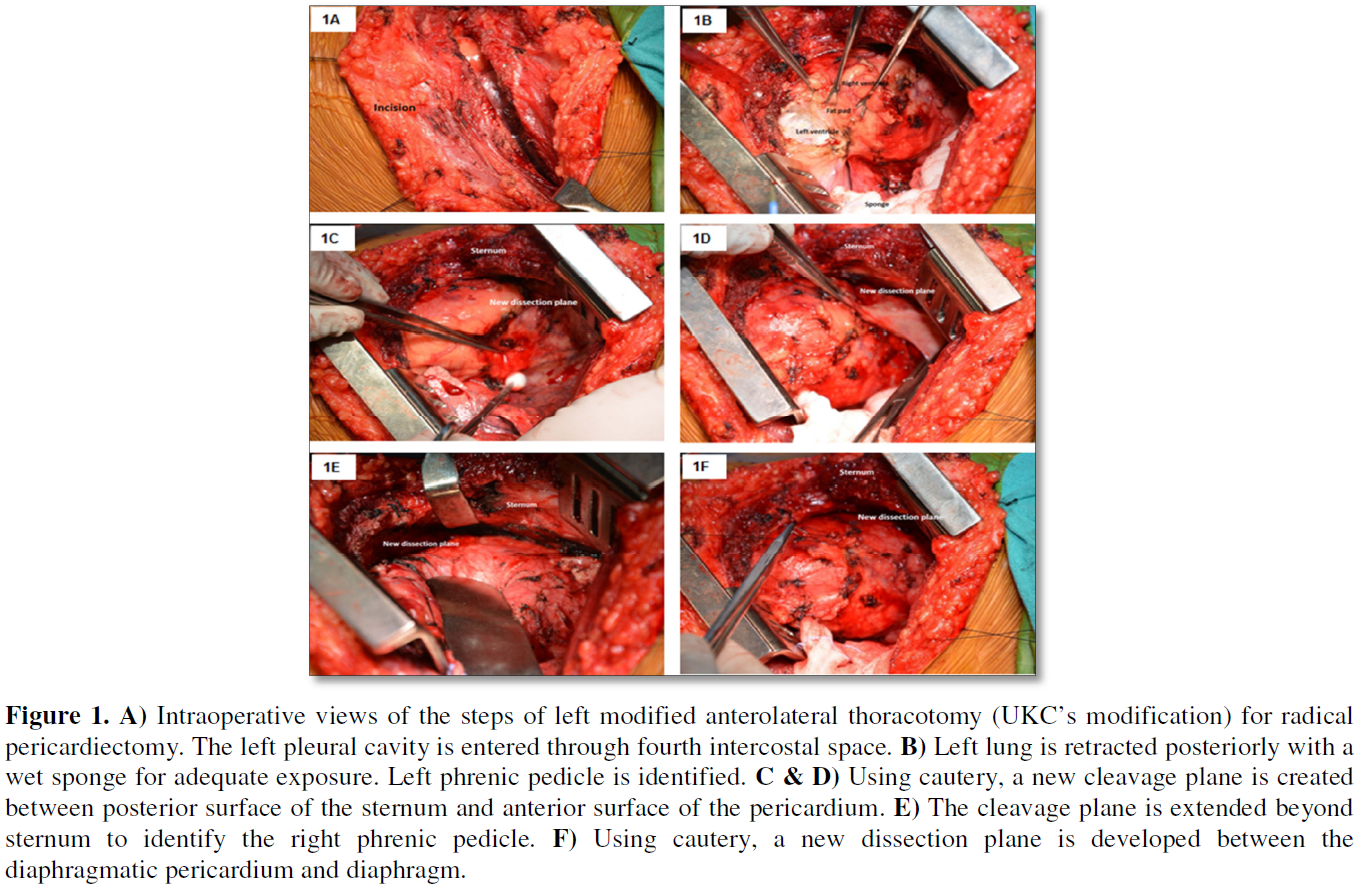
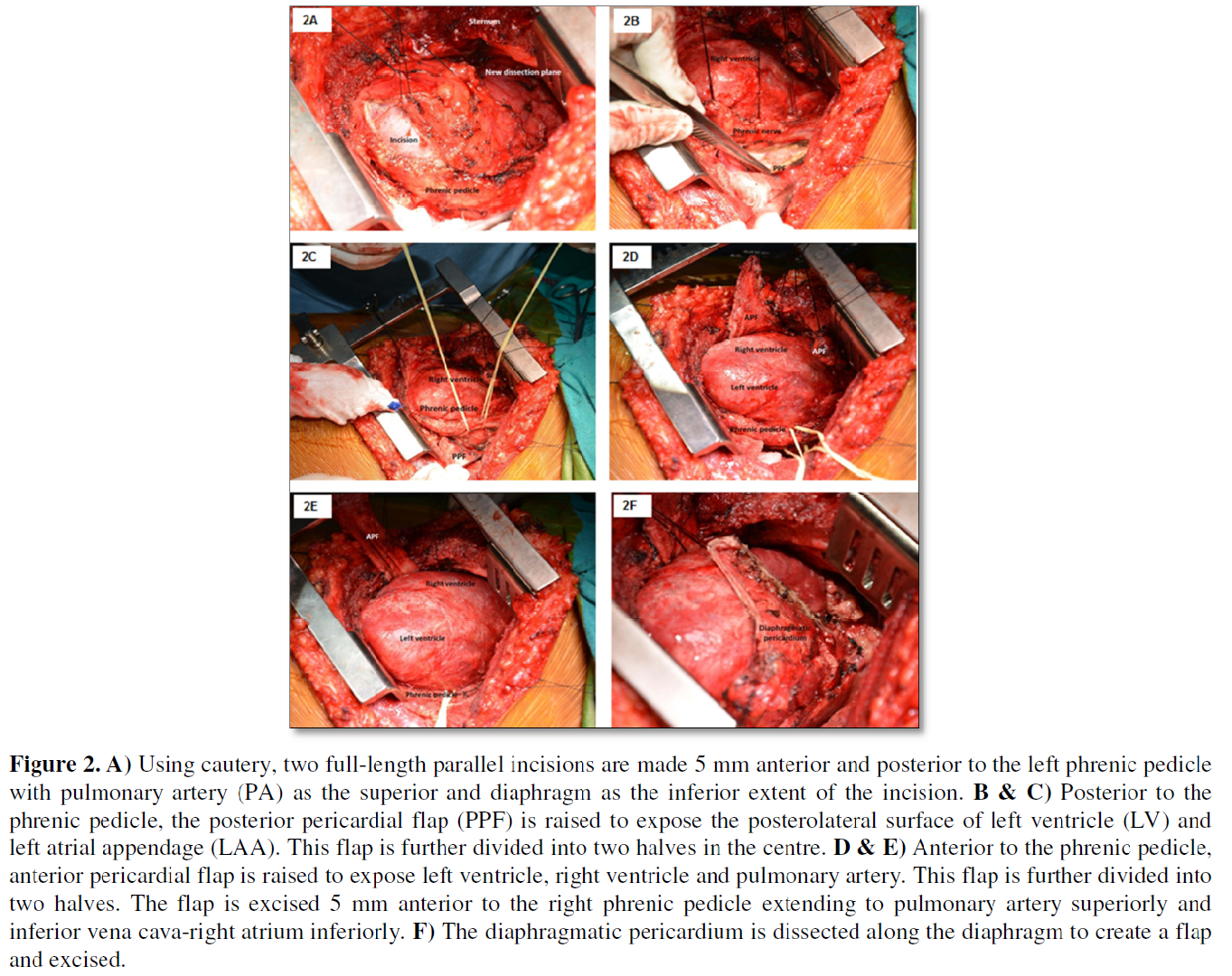
SURGICAL TECHNIQUES AND RESULTS
The step-by-step technical details of the conventional median sternotomy (n=55) and the authors modification of the left anterolateral thoracotomy (n=67) to achieve radical pericardiectomy without utilizing cardiopulmonary bypass have been alluded to in our previous publications [4,6,31]. The following specific maneuvers (Figures 1A-1F and 2A-2F) facilitated performance of radical pericardiectomy via modified left anterolateral thoracotomy:
1. Development of a new cleavage plane between the sternum and the anterior surface of the pericardium using cautery and a right angled deep blade sternal retractor.
2. Extension of the dissection plane beyond the midsternum to the right phrenic pedicle.
3. Development of a new cleavage plane between the diaphragmatic pericardium and diaphragm.
4. Dissection of the pericardium posterior to the left phrenic nerve and division of the posterior pericardium in two halves.
5. Dissection of the pericardium anterior to the phrenic nerve, division of the anterior pericardium in two halves and detachment of the anterior pericardium 1 cm away from the right atrio-ventricular groove.
Using these modifications, radical pericardiectomy was associated with a further reduction of operative mortality as compared to total pericardiectomy of our initial publication (2.9% vs. 7.6%) and patients undergoing total pericardiectomy of our second publication (2.9% vs. 7.2%) [4,6,31]. By employing these modifications, we have been able to reduce the incidence of postoperative LCOS from 69% (total pericardiectomy) to 26.8% (radical pericardiectomy) [4,6,31,33,51].
On the basis of the published literature including ours enunciated in the manuscript, we would recommend the following for the management of LCOS following pericardiostomy and pericardiectomy:
1. Irrespective of the pathologic mechanism of this lethal syndrome, the cardiologist or cardiac surgeon should primarily consider gradual pericardiocentesis to facilitate gradual myocardial adaptation. After achieving improved hemodynamics, complete decompression of the pericardium may be safe and feasible.
2. There are massive fluid shifts with autotransfusion following percardiectomy and massive ascites is a significant negative factor for survival. Intraoperative insertion of a peritoneal dialysis catheter and drainage of the ascitic fluid during pericardiectomy and over next 2-3 h prevents sudden auto-transfusion, increased preload and failure of the Frank Sterling mechanism after surgery.
3. Routine and serial utilization of FloTracTM/VigileoTM device may be the investigation of choice for hemodynamic monitoring of these patients with LCOS following pericardiostomy and pericardiectomy.
4. Monitoring of central venous pressure alone may not suffice for hemodynamic assessment because it is a static preload indicator. Elective use of inotropes like dopamine and dobutamine immediately on completion of pericardiectomy is safer than institution in the late postoperative period. Trial administration of intravenous fluid or blood in these patients may actually be deleterious.
5. Total pericardiectomy through median sternotomy is associated with enhanced safety, decreased mortality less postoperative LCOS, abbreviated hospitalization and better long-term survival than that obtained via thoracotomy. In the event of inadvertent excessive bleeding, the patient can easily be connected to cardiopulmonary bypass.
6. Median sternotomy is the approach of choice for chronic constrictive pericarditis, calcific patches, pericardial masses and redo-pericardiectomy.
7. Left anterolateral thoracotomy should be reserved for surgery for pyogenic and effusive pericardial diseases.
8. Although routine use of cardiopulmonary bypass (CPB) is a subject of debate, one should not be reluctant to utilize CPB if needed in select instances as enumerated in the text to facilitate a complete resection to improve survival. Additionally, the use of CPB allows one to control those fluid shifts and ultrafiltrate some of this fluid off.
9. Radical pericardiectomy via modified left anterolateral thoracotomy without using cardiopulmonary bypass as developed by the authors recently, is associated with a further reduction of operative mortality as compared to total pericardiectomy (2.9% vs. 7.6%) and a further reduction of postoperative LCOS from 69% (total pericardiectomy) to 26.8% (radical pericardiectomy).
10. Successful pericardiectomy requires removal of the pericardium as described under total and radical pericardiectomy including decortication of the ventricular epicardial peel as described by Harrington.
11. It is mandatory to minimize cardiac manipulation and intermittent prolonged hypotension during pericardiectomy to decrease postoperative myocardial edema.
12. Intra-aortic balloon counterpulsation facilitates recovery of ventricular function and appears to be a reasonable alternative in select instances of refractory cardiac failure following pericardiectomy. Timely institution of intra-aortic balloon counterpulsation in patients with sudden deterioration or progressive deterioration of ventricular function, unresponsive to optimal inotropic support is the key for a successful outcome.
CONCLUSION
Low cardiac output syndrome is an expected, frequent physiologic challenge following pericardiostomy and pericardiectomy that requires exquisitely diligent bedside monitoring and thoughtful intervention. The initiation of therapeutic strategies such as inotropes, steroids, inodilators, afterload reducing agents and mechanical ventilation may all have a role in augmenting cardiac output, decreasing oxygen demand, and improving the relationship between oxygen supply and demand. When medical interventions fail, transition to IABC should be pursued to support end organ function, allowing for myocardial recovery.
We advocate caution against wide spread use of balloon pumping after pericardiectomy. Clearly, there must be an exhaustive search for adequacy of pericardiectomy, exclusion of any other surgically correctable cause(s) like significant mitral and/or tricuspid regurgitation, significant coronary artery disease or other correctable surgical issues. In patients undergoing pericardiectomy with left or right ventricular failure which leads to biventricular failure, intra-aortic balloon counterpulsation can be successfully used. Timing of initiation of support remains difficult since its use as an absolute “last resort” decreases the possibility of success. Randomized studies should be performed to define specific indications, proper time of intervention and factors that can predict a successful outcome.
COMPLIANCE WITH ETHICAL STANDARDS
Statement of human rights/ethical approval
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki declaration of 1975, as revised in 2008 and has been approved by the Institutional Research Committee.
1. Rehn S (1935) Chronic constrictive pericarditis (Pick’s disease) treated by pericardial resection. Lancet 2: 539-597.
2. Harrington SW (1944) Chronic constrictive pericarditis. Partial pericardiectomy and epicardiolysis in twenty-four cases. Ann Surg 120: 468-485.
3. Myers RB, Spodick DH (1999) Constrictive pericarditis. Clinical and pathophysiologic characteristics. Am Heart J 138: 219-232.
4. Chowdhury UK, Subramaniam GK, Kumar AS, Airan B, Singh R, et al. (2006) Pericardiectomy for constrictive pericarditis: A clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg 81: 522-529.
5. Maisch B, Severovic PM, Ristic AD, Erbel R, Rienmuller R, et al. (2004) For the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases. Eur Heart J 25: 587-610.
6. Chowdhury UK, Narang R, Malhotra P, Choudhury M, Choudhury A, et al. (2016) Indications, timing and techniques of radical pericardiectomy via modified left anterolateral thoracotomy (UKC’s modification) and total pericardiectomy via median sternotomy (Holman and Willett) without cardiopulmonary bypass. J Prac Cardiovasc Sci 2: 17-27.
7. Seferovic PM, Ristic AD, Imazio M, Maksimovic R, Simeunovic D, et al. (2006) Management strategies in pericardial emergencies. Herz 31: 891-900.
8. McCaughan BC, Schaff HV, Piehler JM, Danielson GK, Orszulak TA, et al. (1985) Early and late results of pericardiectomy for constrictive pericarditis. J Thorac Cardiovasc Surg 89: 340-350.
9. Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y, et al. (2010) Controversial issues in the management of pericardial diseases. Circulation 121: 916-928.
10. Bozbuga N, Erentug V, Eren E, Erdogan HB, Kirali K, et al. (2003) Pericardiectomy for chronic constrictive tuberculous pericarditis. Tex Heart Inst J 30: 180-185.
11. Parr GVS BE, Kirklin JW (1975) Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation 51: 867-874.
12. Wernovsky G, Wypij D, Jonas RA (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92: 2226-2235.
13. Vandyke WH, Cure J, Chakko CS, Gheorghiade M (1983) Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 309: 595-596.
14. Wolfe MW, Edelman ER (1993) Transient systolic dysfunction after relief of cardiac tamponade. Ann Intern Med 119: 42-44.
15. Spodick DH (1983) The normal and diseased pericardium: Current concepts of pericardial physiology, diagnosis and treatment. J Am Coll Cardiol 1: 240-251.
16. Merce J, Sagrista-Sauleda J, Permanyer-Miralda G, Soler-Soler J (1998) Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am J Med 105: 106-109.
17. Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, Soler-Soler J (2000) Clinical clues to the causes of large pericardial effusions. Am J Med 109: 95-101.
18. Clare GC, Troughton RW (2007) Management of constrictive pericarditis in the 21st century. Curr Treat Options Cardiovasc Med 9: 436-442.
19. Schwefer M, Aschenbach R, Heidemann J, Mey C, Lapp H, et al. (2009) Constrictive pericarditis, still a diagnostic challenge: Comprehensive review of clinical management. Eur J Cardiothorac Surg 36: 502-510.
20. Tokuda Y, Miyata H, Motomura N, Araki Y, Oshima H, et al. (2013) Outcome of pericardiectomy for constrictive pericarditis in Japan: A nationwide outcome study. Ann Thorac Surg 96: 571-576.
21. Zhu P, Mai M, Wu R, Lu C, Fan R, et al. (2015) Pericardiectomy for constrictive pericarditis: Single-centre experience in China. J Cardiothorac Surg 10: 34.
22. Sabzi F, Faraji R (2015) Predictors of post pericardiotomy low cardiac output syndrome in patients with pericardial effusion. J Cardiovasc Thorac Res 7: 18-23.
23. Chandler HK, Kirsch R (2016) Management of the low cardiac output syndrome following surgery for congenital heart disease. Curr Cardiol Rev 12: 107-111.
24. Gregory JR, McMurtrey MJ, Mountain CF (1985) A surgical approach to the treatment of pericardial effusion in cancer patients. Am J Clin Oncol 8: 319-323.
25. Dosios T, Angouras D (1997) Low cardiac output syndrome complicating subxiphoid pericardiostomy for pericardial effusion. J Thorac Cardiovasc Surg 113: 220.
26. Moores DWO, Allen KB, Faber LP, Dziuban ST, Gillman DJ, et al. (1995) Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg 109: 546-552.
27. Palatianos GM, Thurer RJ, Pompeo MQ, Kaiser GA (1989). Clinical experience with subxiphoid drainage of pericardial effusions. Ann Thorac Surg 48: 381-385.
28. Sunday R, Robinson LA, Bosek V (1999). Low cardiac output complicating pericardiectomy for pericardial tamponade. Ann Thorac Surg 67: 228-231.
29. Mayosi BM, Wiysonge CS, Ntsekhe M, Volmink JA, Gumedze F, et al. (2006) Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: The investigation of the management of pericarditis in Africa (IMPI Africa) registry. BMC Infect Dis 6: 2.
30. Chowdhury UK, Kapoor PM, Rizvi A, Malik V, Seth S, et al. (2017) Serial semi-invasive hemodynamic assessment following pericardiectomy for chronic constrictive pericarditis. Ann Card Anesth 20: 169-177.
31. Chowdhury UK, Seth S, Reddy SM (2008). Pericardiectomy for chronic constrictive pericarditis. J Operative Tech Thorac Cardiovasc Surg 13: 14-25.
32. Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, et al. (1999) Constrictive pericarditis in the modern era: Evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 100: 1380-1386.
33. Chowdhury UK, Kumari LS, Hasija S (2018) Surgery for chronic constrictive pericarditis, tuberculous pericarditis and effusive-constrictive pericarditis. Cardiological Society of India, 2018. Essentials of Postgraduate Cardiology, Evangel Publishers, Invited Chapter 64, pp: 1-10.
34. Chowdhury UK, Kumari LS (2018) Pericardiectomy for chronic constrictive pericarditis: Where are we after 100 years? World J Surg Surg Res 1: 1027-1030.
35. Braverman AC, Sundaresan S (1994) Cardiac tamponade and severe ventricular dysfunction. Ann Intern Med 120: 442.
36. Chamoun A, Cenz R, Mager A, Rahman A, Champion C, et al. (2003) Acute left ventricular failure after large volume pericardiocentesis. Clin Cardiol 26: 5880-5890.
37. Robinson LA, Ruckdeschel JC (1998) Management of pleural and pericardial effusions. In: Berger A, Portenoy RK, Weissman DE. Principles and practices of supportive oncology. 1st Edn. Philadelphia: Lippincott-Raven, pp: 327-352.
38. Glasser F, Fein AM, Feinsilver SH, Cotton E, Niederman MS, et al. (1988) Non-cardiogenic pulmonary edema after pericardial drainage for cardiac tamponade. Chest 94: 869-870.
39. Hamaya Y, Dohi S, Ueda N, Akamatsu S (1993) Severe circulatory collapse immediately after pericardiocentesis in a patient with chronic cardiac tamponade. Anesth Analg 77: 1278-1281.
40. Wechsler AS, Auerbach BJ, Graham TC, Sabiston DC (1974). Distribution of intramyocardial blood flow during pericardial tamponade. Correlation with microscopic anatomy and intrinsic myocardial contractility. J Thorac Cardiovasc Surg 68: 847-856.
41. Ligero C, Leta R, Bayes-Genis A (2006) Transient biventricular dysfunction following pericardiocentesis. Eur J Heart Fail 8: 102-104.
42. Skalidis EI, Kochiadakis GE, Chrysostomakis SI, Igoumenidis NE, Manios EG, et al. (2000) Effect of pericardial pressure on human coronary circulation. Chest 117: 910-912.
43. Douglas JM (1995) The pericardium. In: Sabiston DC, Spencer FC. Surgery of the chest. 6th Edn. Philadelphia: WB Saunders, pp: 1365-1386.
44. Dines DE, Edwards JE, Burchell HB (1958) Myocardial atrophy in constrictive pericarditis. Staff Meetings Mayo Clin 33: 99-93.
45. Johnson TL, Bauman WB, Josephson RA (1993) Worsening tricuspid regurgitation following pericardiectomy for constrictive pericarditis. Chest 104: 79-81.
46. Levine HD (1973) Myocardial fibrosis in constrictive pericarditis electrocardiographic and pathologic observations. Circulation 48: 1268-1281.
47. Roberts JT, Beck CS (1941). The effect of chronic cardiac compression on the size of the heart muscle fibers. Am Heart J 22: 314-319.
48. Connors AF, McCaffree DR, Gray BA (1983) Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N Engl J Med 308: 263-267.
49. Lobos AT, Lee S, Menon K (2012) Capillary refill time and cardiac output in children undergoing cardiac catheterization. Pediatr Crit Care Med 13: 136-140.
50. Oh JK, Hatle LK, Seward JB, Danielson GK, Schaff HV, et al. (1994) Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol 23: 154-162.
51. Chowdhury UK, Patel K, Kumari L, Seth S, Avneesh S, et al. (2019) Tissue Doppler imaging-derived mitral and tricuspid annular velocities: Non-predictors of operative outcome in patients undergoing pericardiectomy for chronic constrictive pericarditis. J Cardiol Diagn Res 2: 67-83.
52. Senni M, Redfield MM, Ling LH, Danielson GK, Tajik AJ, et al. (1999) Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis: Doppler echocardiographic findings and correlation with clinical status. J Am Coll Cardiol 33: 1182-1188.
53. Chandler HK, Kirsch R (2016) Management of the low cardiac output syndrome following surgery for congenital heart disease. Curr Cardiol Rev 12: 107-111.
54. Kloster FR, Crislip RL, Bristow JD, Herr RH, Ritzmann LW, et al. (1962). Hemodynamic studies following pericardiectomy for constrictive pericarditis. Circulation 25: 484.
55. Fitzpatrick DP, Wyso EM, Bosher LH, Richardson DW (1962) Restoration of normal intracardiac pressures after extensive pericardiectomy for constrictive pericarditis. Circulation 25: 484-492.
56. Harrison EC, Crawford DW, Lan FYK (1970) Sequential left ventricular function before and after pericardiectomy for constrictive pericarditis. Am J Cardiol 26: 319-323.
57. Sandham JD, Hull RD, Brant RF (2003). A randomized, controlled trial of the use of pulmonary artery catheters in high-risk surgical patients. N Engl J Med 348: 5-14.
58. Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, et al. (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomized controlled trial. Lancet 366: 472-477.
59. Baulig W, Bernhard EO, Bettex D, Schmidlin D, Schmid ER, et al. (2005) Cardiac output measurement by pulse dye densitometry in cardiac surgery. Anesthesia 60: 968-973.
60. Rodig G, Prasser C, Keyl C, Liebold A, Hobbhahn J, et al. (1999) Continuous cardiac output measurement: Pulse contour analysis vs. thermodilution technique in cardiac surgical patients. Br J Anesth 82: 525-530.
61. Gaines WE, Pierce WS, Prophet GA, Holtsman K (1984) Pulmonary circulatory support: a quantitative comparison of four methods. J Thorac Cardiovasc Surg 88: 958-964.
62. Della Rocca G, Costa MG, Pompel L, Coccia C, Pietropaoli P, et al. (2002) Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anesth 88: 350-356.
63. Manecke GR (2005) Edwards FloTrac™ sensor and Vigileo™ monitor: Easy, accurate, reliable cardiac output assessment wave. Expert Rev Med Devices 2: 523-527.
64. Reuter DA, Felbinger TW, Schimdt C, Kilger E, Goedje O, et al. (2002) Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med 28: 392-398.
65. Osman D, Ridel C, Ray P, Monnet X, Anguel N, et al. (2007) Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 35: 1-5.
66. Rex S, Brose S, Metzelder S (2004) Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anesth 93: 782-788.
67. Bailey JM, Miller BE, Lu W, Tosone SR, Kanter KR, et al. (1999) The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology 90: 1012-1018.
68. Chang AC, Atz A, Wernovsky G, Burke RP, Wessel DL, et al. (1995) Milrinone: Systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med 23: 1907-1914.
69. Hoffman T, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, et al. (2003) Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 107: 996-1002.
70. Wright EM SJ, Sherry KM (1992) Milrinone in the treatment of low output states following cardiac surgery. Eur J Anesthesiol, pp: 21-26.
71. Pathak A, Lebrin M, Vaccaro A, Senard JM, Despas F (2013) Pharmacology of levosimendan: Inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther 38: 341-349.
72. Toller W, Heringlake M, Guarracino F, Algotsson L, Alvarez J, et al. (2015) Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int J Cardiol 184: 323-336.
73. Gillies M, Bellomo R, Doolan L, Buxton B (2005) Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery - A systematic literature review. Crit Care 9: 266-279.
74. Alten JA, Borasino S, Toms R, Law MA, Moellinger A, et al. (2012) Early initiation of arginine vasopressin infusion in neonates after complex cardiac surgery. Pediatr Crit Care Med 13: 300-304.
75. Burton GL, Kaufman J, Goot BH, da Cruz EM (2011) The use of arginine vasopressin in neonates following the Norwood procedure. Cardiol Young 21: 536-544.
76. Mastropietro CW, Davalos MC, Seshadri S, Walters HL 3rd, Delius RE, et al. (2013) Clinical response to arginine vasopressin therapy after pediatric cardiac surgery. Cardiol Young 23: 387-393.
77. Mastropietro CW, Rossi NF, Clark JA (2010) Relative deficiency of arginine vasopressin in children after cardiopulmonary bypass. Crit Care Med 38: 2052-258.
78. Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, et al. (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95: 1122-1125.
79. Menon K (2013) Use of hydrocortisone for refractory shock in children. Crit Care Med 41: 294-295.
80. Shore S, Nelson DP, Pearl JM, Manning PB, Wong H, et al. (2001) Usefulness of corticosteroid therapy in decreasing epinephrine requirements in critically ill infants with congenital heart disease. Am J Cardiol 88: 591.
81. Suominen PK, Dickerson HA, Moffett BS, Ranta SO, Mott AR, et al. (2005) Hemodynamic effects of rescue protocol hydrocortisone in neonates with low cardiac output syndrome after cardiac surgery. Pediatr Crit Care Med 6: 655-659.
82. Verweij EJ, Hogenbirk K, Roest AA (2012) Serum cortisol concentration with exploratory cut-off values do not predict the effects of hydrocortisone administration in children with low cardiac output after cardiac surgery. Int Cardiovasc Thorac Surg 15: 685-689.
83. Costello JM, Graham DA, Morrow DF, Potter-Bynoe G, Sandora TJ, et al. (2009) Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med 10: 453-459.
84. Mastropietro CW, Barrett R, Davalos MC (2013) Cumulative corticosteroid exposure and infection risk after complex pediatric cardiac surgery. Ann Thorac Surg 95: 2133-2139.
85. Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, et al. (2010) Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation 122: 2123-2130.
86. Viires N, Sillye G, Aubier M, Rassidakis A, Roussos C, et al. (1983) Regional blood flow distribution in dog during induced hypotension and low cardiac output. J Clin Invest 72: 935-947.
87. Ha JW, Oh JK, Schaff HV, Ling LH, Higano ST, et al. (2008) Impact of left ventricular function on immediate and long-term outcomes after pericardiectomy in constrictive pericarditis. J Thorac Cardiovasc Surg 136: 1136-1141.
88. Romeo FJ, Guzzetti E, Arias A, Belziti C, Marenchino R (2015) New-onset liver failure: Pitfalls of an unusual diagnosis. Arch Cardiovasc Imaging 3: e33652.
89. Wood DE, Crumbley AJ, Pereira NL (2002) Reversible left ventricular dysfunction simulating a myocardial infarction after pericardiectomy. Heart 88: 183-184.
90. Omoto T, Minami K, Varvaras D, Bothig D, Korfer R (2001) Radical pericardiectomy for chronic constrictive pericarditis. Asian Cardiovasc Thorac Ann 9: 286-290.
91. Ruiz-Cano MJ, Fernandez-Ruiz M, Sanchez V, Lopez-Medrano F (2012) Constrictive pericarditis due to Candida albicans: An unexpected cause of pericardial effusion after heart transplantation. Rev Clin Esp 212: 551-557.
92. Kiley S, Sofia J, Machuca T (2017) Venoarterial ECMO for recovery from right ventricular failure after pericardiectomy. SOCCA Post Session 2017 (Abstract), No. 1344.
93. Chowdhury UK, Jena JK, Hasija S, Sankhyan L, et al. (2018) Successful use of intra-aortic balloon counterpulsation for systemic ventricular failure following total pericardiectomy for calcific chronic constrictive pericarditis. World J Pediatr Congenital Heart Surg.1-4, (Available Online).
94. Pinkey KA, Minich LL, Tani LY (2002) Current results with intra-aortic balloon pumping in infants and children. Ann Thorac Surg 13: 887-891.
95. Culliford AT, Lipton M, Spencer FC (1980) Operation for chronic constrictive pericarditis do the surgical approach and degree of pericardial resection influence the outcome significantly? Ann Thorac Surg 29: 146-152.
96. Copeland JG, Riley JE, Fuller J (1986) Pericardiectomy for effusive constrictive pericarditis after heart transplantation. J Heart Transplant 5: 171-172.
97. Copeland JG, Stinson EB, Griepp RB, Shumway NE (1975) Surgical treatment of chronic constrictive pericarditis using cardiopulmonary bypass. J Thorac Cardiovasc Surg 69: 236-238.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)